A woman whose ruptured breast implants were filled with industrial-grade silicone experienced severe breast pain and autoimmune diseases, says the surgeon who successfully removed them while preventing their toxic content from seeping into her body.
Explant surgeon Joshua Lampert, MD, says the patient, who flew from Virginia to Miami for surgery, had been given a brand of breast implants in Columbia that are no longer produced or available worldwide.
Their manufacturer, PIP (Poly Implant Prothèse), was liquidated in 2010, and its founder sentenced to four years in prison in 2013.
PIP was found to be using industrial-grade silicone in its implants, which was 1/10th the cost of approved medical-grade silicone.
A pre-operative MRI showed that one of the woman’s breast implants were ruptured.
In fact, both were.
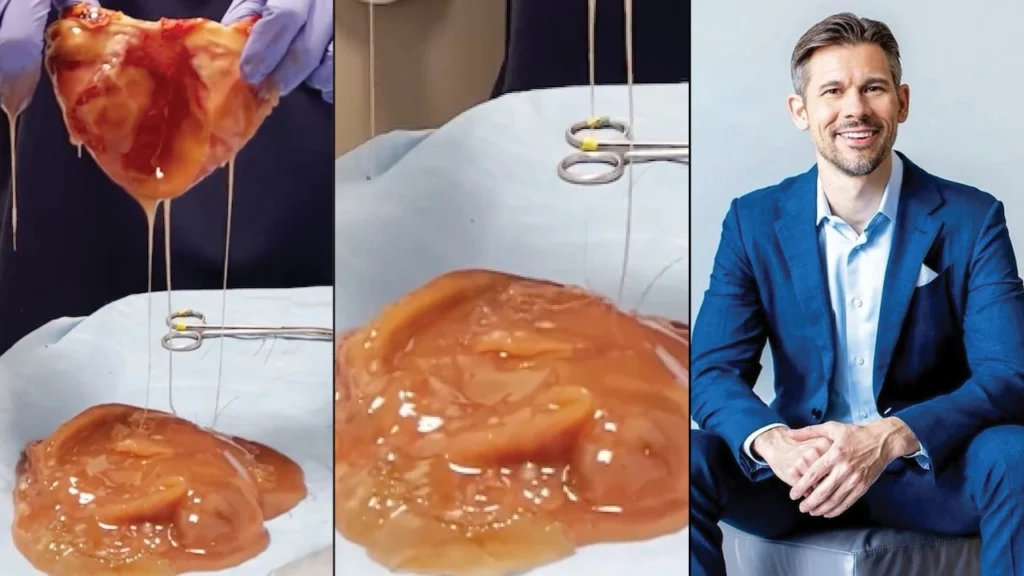
The surgeon says he was able to remove both ruptured implants while preventing the industrial-grade silicone from spilling into her body.
The surgical technique, commonly known as an en bloc capsulectomy, has been called into question by some surgeons.
Explant specialists and patient advocates stand by the procedure, many citing the rare cancers that form in the capsule that naturally forms around all breast implants.
Despite PIP breast implants having been recalled 14 years ago in 2010—and never having been FDA approved for use in the United States—the Miami-based explant surgeon says he still sees a lot of patients who to this day have the defunct and potentially dangerous implants in their body.
