According to the American Society of Plastic Surgeons (ASPS) annual plastic surgery statistics report, $16.7 billion was spent on cosmetic procedures in the U.S. in 2020.1
Many of these procedures involve medical devices and pharmaceutical products. Physicians routinely receive substantial compensation from medical device manufacturers and pharmaceutical companies in the form of gifts, travel, royalties, research grants, and consulting contracts.
“It is time to bring the patient back to the center of the healthcare team.”
In a 2008 Health and Human Services Senate Hearing, Gregory E. Demske, from the Office of Inspector General, testified to the U.S. Senate regarding the financial relationships between physicians and the medical device industry.
In his testimony he states, “scientific evidence suggests that there is a significant risk that payments will improperly influence medical decision making.
Researchers reporting in medical journals, such as the Journal of the American Medical Association and the New England Journal of Medicine, have found that such financial industry-physician relationships are pervasive and the impulse to reciprocate for even small gifts has a powerful influence on behavior.”2
The global medical device market was valued at $412.4 billion in 2020, with $160.1 billion in North America.3 Global aesthetic medical devices are projected to be valued at $15.1 billion in 2024, with North America dominating the market. Two of the top key players in the aesthetic device industry are Abbvie (Allergan) and Johnson & Johnson (Mentor). These companies are also two of the top breast implant manufacturers.
With such a large amount of money at stake, it goes without saying that medical devices and cosmetic procedures are both highly lucrative businesses. Combine the two together and there is big money involved, really big money.
Let’s examine one of the leading breast implant manufacturers and the money trail that leads to a group of physicians who have strategically placed themselves in positions where their influence has controlled the narrative of patient safety and informed consent practices for decades.
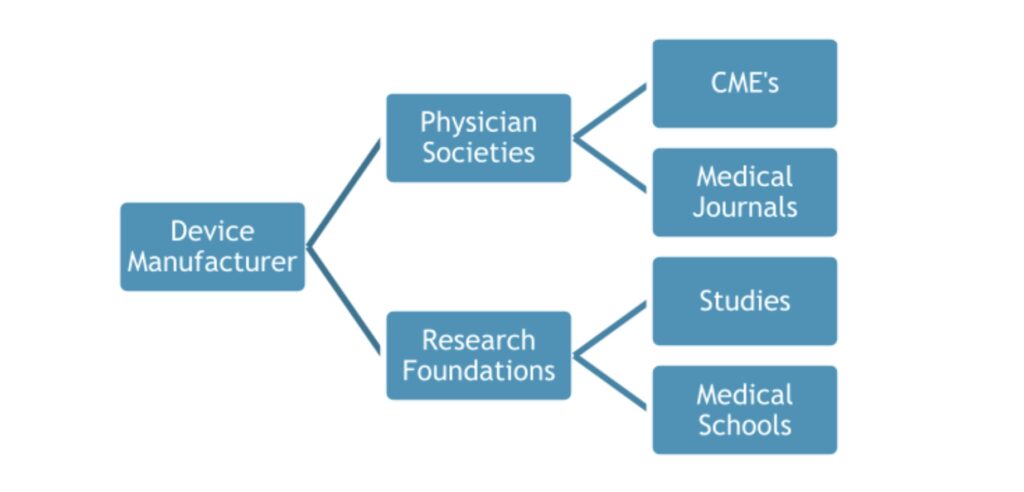
A small group of plastic surgeons and their society leaders have played a key role in promoting the use of breast implants and how the public views the safety of breast implants. These surgeons have extremely close ties to the medical device industry, accepting hundreds of thousands of dollars from the device manufacturers in the form of gifts, travel, food and beverage, consulting fees and speaking engagements.4,5 They testified at FDA hearings in the early 2000’s in favor of allowing silicone breast implants back on the market, even bringing the largest number of patients to testify on behalf of breast implants.6
They were the principal investigators of the clinical trials for one of the world’s most popular textured breast implant, Allergan 410 textured implant, which has now been recalled worldwide for causing a cancer referred to as BIA-ALCL (Breast Implant Associated Anaplastic Large Cell Lymphoma).7 The group has also strategically placed themselves in positions that allow them to control the narrative of all things related to breast implants. This is an industrial conflict of interest, as well as an ethical and moral concern for the health and safety of patients.
“A small group of plastic surgeons and their society leaders have played a key role in promoting the use of breast implants and how the public views the safety of breast implants.”
Robyn Towt, Global Patient Advocacy Coalition
The small group of key players hold positions on the executive boards of medical societies, research foundations, CME companies and medical journals.8 This allows them the ability to control the flow of information that is being disseminated throughout the medical community. For example, if a physician submits an abstract to a medical journal that shows patients have been harmed by breast implants, the doctor who sits on the editorial board of that journal has the power and influence to decide if that particular study should be published. When receiving hundreds of thousands of dollars from industry, it is easy to assume that this doctor may not accept the study due to inherent bias that comes from being closely aligned with the manufacturers.
Similarly, if a physician submits a grant proposal to study harmful effects of breast implants in patients, the same doctor sits on the executive board of the research foundation and may decide that this particular study should not receive the grant. Another example of where we can find bias is in the presentations that are used for CME (Continuing Medical Education) credits and speaking engagements at medical schools.
These doctors are members of the review board that determines which presentations are accepted for CME credit, therefore controlling the information that is taught to medical professionals. In addition, these presentations are typically sponsored and supported by “educational grants” from the device manufacturer.9 It is important to be cautious of studies and presentations that are conducted by doctors who have a blatant conflict of interest, as the study results and information presented can be biased because it is funded by industry.
Conflict of interest in the medical community has been a problem for decades. There has been enough concern that the federal government passed a law in 2010 called The Sunshine Act. The law is designed to reduce inappropriate incentives to physicians to prescribe particular drugs or use certain devices, which may result in added costs for health care and sometimes unnecessary treatment for patients.
The federal government developed a website called Open Payments, where physicians are federally mandated to disclose financial relationships with industry. Another website, ProPublica, has a Dollars for Docs search tool that collects payment reports and compiles them into a database that analyzes the data and compares each doctor to peers in their same specialty and state.
These helpful search tools can be used to obtain valuable information about what types of payments a doctor has received. ProPublica reports that a number of studies have found a link between the drugs and devices doctors choose to use with their patients and payments they receive from drug makers and device makers.10,11
“Surgical specialists received seven times more money from device firms than drug vendors.”
In a STAT News report dated April 6, 2021, it was reported that the medical device industry gave doctors incentive payments worth $904 million between 2014 and 2017. It is stated that researchers found that the device industry provided the most payments to surgical specialists. Surgical specialists received seven times more money from device firms than drug vendors. Doctors who receive these payments are more likely to implant or otherwise use the manufacturer’s medical devices in their practice.”12
It is concerning to know that the medical device companies have such a large influence in the medical community. In any healthcare setting, the patient should be the top priority, at the center of the health care team. Unfortunately, this is not always the case, and patients who are experiencing this conflict of interest in health care are speaking out.
Healthcare has become a multi-billion dollar business that is run for economic gain, and somewhere along the line the patient has been forgotten. However, patients who are frustrated with sub-par standard of care are speaking out on social media and other platforms demanding accountability, transparency, and proper informed consent.
Doctors who are providing excellent patient centered care are becoming increasingly in high demand, which will organically improve the standard of care as the less patient centered doctors will lose customers. Ultimately, the medical device companies will no longer have use for doctors who have a low volume of patients. Patients will drive the standard of care, and hopefully bring the medical experience back to being patient centered instead of profit driven
It is important for patients to research a doctor or surgeon to find out what their conflicts of interest may be. Fortunately, most patients are able to choose a different doctor if they do not find the right fit. It is time to bring the patient back to the center of the healthcare team.
Robyn Towt
Global Patient Advocacy Coalition
www.gpacunited.org
Robyn Towt is a former teacher and three-time cancer survivor who was harmed by a medical device, leading to her passion for advocacy in medical device safety. She is a co-founder of the Global Patient Advocacy Coalition and a member of the Arizona Association of Patient Advocates. She has testified twice at the FDA in support of patient safety and has worked extensively with Arizona legislators in passing an informed consent law for breast implant surgery.
