Patients, advocates, and colleagues lauded a plastic surgeon who raised concerns Tuesday about the industry’s financial arrangements, questioning conflicts of interest.
The doctor said colleagues had asked why he didn’t attend the latest “big meeting”—a lavish five-day event hosted by The Aesthetic Society in Miami from April 19th – 23rd.
“Truth is, I have lost faith in much of our industry, our societies, our thought leaders,” Dr. Christian Subbio shared with 148,000 followers on social media.
At issue, according to the Philadelphia based board-certified plastic surgeon, is that he believes institutions trusted by patients and the public to create unbiased science are on industry or manufacturer payrolls “to an astonishing extent.”
“From cash honorariums, to fancy receptions, to free trips, to academic grants, to stock options, I’m not quite sure how this is allowed or tolerated, but it needs to change,” the surgeon shared.
“At the very least these ‘generous’ contributions from big Pharma companies and device companies need to be made more transparent.”

Payments to physicians from drug and device manufacturers are publicly available at OpenPayments.gov.
In 2021, the latest year for which data is available, $10.88 billion in payments are recorded.
Providers are “strongly encouraged” to review and dispute the data prior to its publication, which usually occurs 6 months after the year payments were made.
Financial disclosures for those who served as faculty at The Aesthetic Meeting were made available on a meeting website.
One hundred thirty five faculty members listed “nothing to disclose.”
Others listed as many as eight disclosures.
In addition to faculty disclosures, the Society made publicly available a list of its industry partnerships, prior to its Miami meeting.

Most plastic surgeons who weighed in agreed there was room for improvement—many dramatically so.
Others say the meetings are a necessary part of advancing the industry and that panelists actually lose revenue when attending, despite industry sponsorships.
At the 23rd hour of a public poll hosted by the surgeon, 95% of an unknown number of respondents voted “Yes” when asked, “Are the medical aesthetics and its leaders compromised by big pharma and device money?”
Aesthetic Society leadership and media contacts didn’t respond to three emails requesting comment.
The Society’s immediate past president and one of its three media contacts, Dr. Jennifer Walden, is co-author of an industry book chapter on media relations.
Walden advises colleagues that “not all media is good media,” and to “choose media outlets carefully.”
Surgeons should “consider saying ‘no’” when being asked to comment on studies, procedures, and technologies that are “highly controversial,” she says.
The Philadelphia surgeon isn’t the first to raise concern over the industry’s financial arrangements, or The Aesthetic Society.
Dr. Diana Zuckerman, founder of the National Center for Health Research, cautioned patients in August against relying on information from the Society, after it published what Zuckerman suggested “may be the worst” study of its kind ever published.
Zuckerman concluded in summary that an April 2022 article from the Society, “makes it clear that the Aesthetic Society is encouraging their members to ‘gaslight’ patients with BII, rather than help them get explanted.”
“All but one of the authors have financial ties to companies that make breast implants.”
Dr. Diana Zuckerman, on a Breast Implant Illness study published by The Aesthetic Surgery Journal.
The Aesthetic Society subsequently granted the article’s authors its Award of Excellence, for “Best Journal Article,” and granted its Tiffany Award to a presentation discussing the study.
In its Miami meeting, Wednesday, two of the article’s five authors were elected to leadership roles.
Co-author Melinda Haws, M.D., was named the new president of The Aesthetic Society.
Author Patricia McGuire, M.D., was named the new Vice President of ASERF, the Society’s “Aesthetic Surgery Education and Research Foundation.”
(ASERF’s outgoing President, Dr. Bruce Van Natta, presumably viewed patient BII groups with almost total disdain.)
Among the many patients weighing in on the discussion of industry conflicts—and reposting the doctor’s statements to their own social media accounts—were patient advocates and women with breast implant illness—the very people who should find consolation in advances toward healing.
Yet many indicate they don’t, citing such facts as the FDA asking one manufacturer to voluntary recall a breast implant from market after studies linked it to increased risks of cancer.
In its heyday, that implant, one advocate says, was hailed as the newest, greatest, and safest of its kind; the result of 10 more years of research.
At least two videos of surgeon’s celebrating the arrival of that now-recalled implant have been removed from the Internet.
They featured surgeons who are current co-authors of studies on breast implant illness.
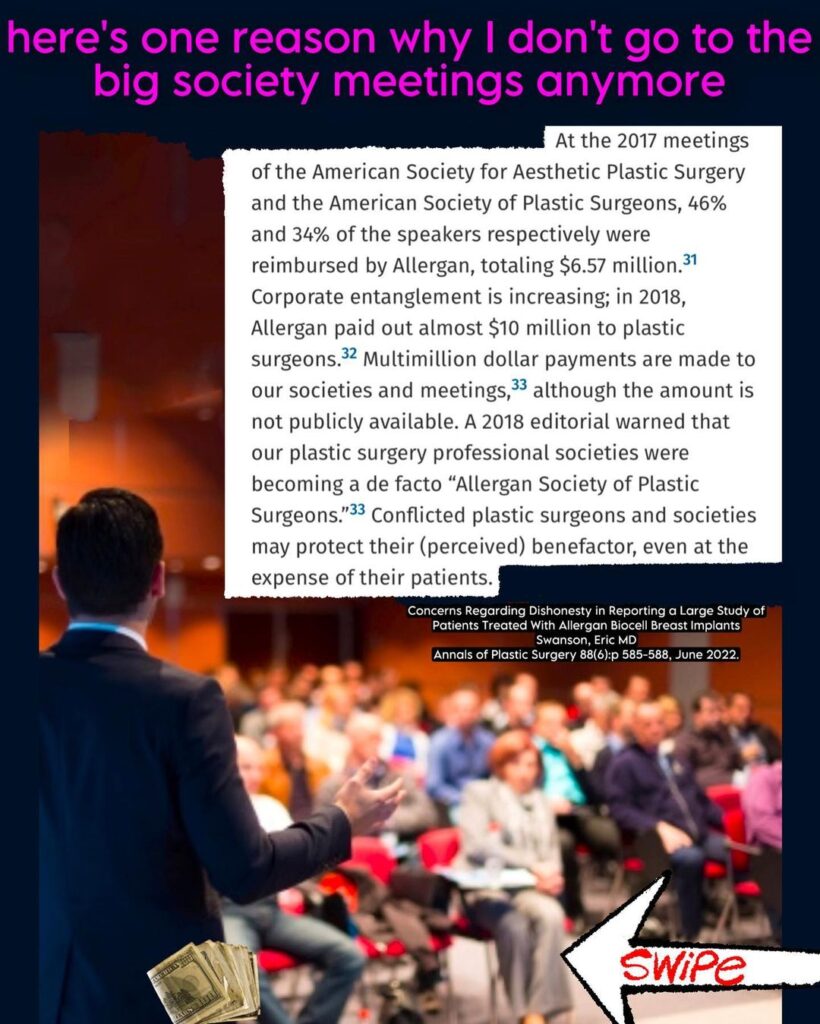
On Tuesday, Dr. Christian Subbio cited and shared a portion of a June 2022 editorial by Dr. Eric Swanson.
Swanson detailed the extent of influence by device manufacturers on members of the industry and its conferences.
“At the 2017 meetings of the American Society for Aesthetic Plastic Surgery and the American Society of Plastic Surgeons, 46% and 34% of the speakers respectively were reimbursed by Allergan, totaling $6.57 million….
“A 2018 editorial warned that our plastic surgery professional societies were becoming a de facto ‘Allergan Society of Plastic Surgeons,’” a shared portion of the editorial says.
Intentional or not, the Swanson editorial shared, linked, at least in part, back to Aesthetic Society leadership.
“Six of the 8 authors of the 2017 study disclosed that they were Allergan consultants, including [lead author Bill] Adams, who denied financial conflicts at the FDA hearing, despite a publication disclosing his conflicts with Allergan and Sientra. According to the Dollars for Docs Web site, Adams received more than US $100,000 from Allergan between 2016 and 2018,” Swanson writes.
Adams, a recent past president of the Society, is today listed as one of its three media contacts, from whom we never heard.
Plastic surgery societies suggest industry partnerships “advance the science, art, and safe practice” of cosmetic surgery through education, research and innovation.
This plastic surgeon, and many patients, suggest they believe otherwise.
Board-certified Nevada-based plastic surgeon, Dr. Christopher Costa, M.D., called the surgeon’s points eye-opening and concerning.
“The truth is that we would not be able to have these meetings without industry support, but direct (or indirect) payment to the people writing the studies completely taints the validity of the work that is being done.”
Dr. Heidi A Waldorf, M.D., says contrary to claims of financial benefit, most physician speakers actually lose revenue during industry events, by not being at their practices.
Waldorf, who supports industry meetings and offered multiple counterpoints to Dr. Subbio’s concerns, believes that both patients and providers benefit in ways despite the lost revenue.
“BUT by being there—by teaching and sharing ideas—getting our brains percolating—we bring innovative ideas back to our offices and our patients benefit,” Waldorf wrote.
(Waldorf, according to her website, is “an investigator, speaker, consultant, advisory board member and trainer for major industry producers of drugs, devices and cosmeceuticals.”)
Yet one surgeon suggests such innovative ideas aren’t welcome at industry meetings.
Dr. Sheila Nazarian, a board-certified Beverly Hills plastic surgeon, says she stopped attending some industry events “because of the bashing of real innovators.”
“Anyone who dares pop their head above the crowd is promptly smacked down. Like aesthetic whac-a-mole,” Nazarian wrote.
At press time, the surgeon’s statement had received nearly 5,000 likes and 470 comments.
